Last updated on July 14th, 2024 at 05:42 pm
Haloalkanes and Haloarenes MCQ Chapter 10
The Haloalkanes are a group of chemical compounds having an alkane with one or more hydrogen replaced by a halogen atom (group 17 elements). The difference between an alkane and haloalkane is quite significant.
The structural difference is because of the replacement of one or more hydrogen with a halogen atom. The difference in physical property is due to the difference in the electronegativity, bond length, bond strength, molecular size etc.
Haloarenes are aromatic compounds in which the halogen atom attaches directly to the carbon atom of the aromatic ring.
Below are some of the very important NCERT Haloalkanes and Haloarenes MCQ Class 12 Chemistry Chapter 10 with Answers. These Haloalkanes and Haloarenes MCQs have been prepared by expert teachers and subject experts based on the latest syllabus and pattern of CBSE Term 1 examination.
We have given these Haloalkanes and Haloarenes Class 12 Chemistry MCQs Questions with Answers to help students understand the concept.
MCQ Questions for Class 12 Chemistry are very important for the latest CBSE Term 1 and Term 2 pattern. These MCQs are very important for students who want to score high in CBSE Board, NEET and JEE exam.
We have put together these NCERT MCQ Questions of Haloalkanes and Haloarenes for Class 12 Chemistry Chapter 10 with Answers for the practice on a regular basis to score high in exams. Refer to these MCQs Questions with Answers here along with a detailed explanation.
Note : – Answers after every 10 questions


Haloalkanes and Haloarenes MCQ (1-10)
- In the presence of peroxide hydrogen chloride and hydrogen iodide do not give anti-Markovnikov’s addition to alkenes because
- Both are highly ionic
- One is oxidising and the other is reducing
- One of the steps is endothermic in both the cases
- All the steps are exothermic in both the reactions
- On being heated with aniline and KOH, chloroform gives
- An almond like smell
- A rose-like odor
- A smell like oil of wintergreen
- An offensive smell
- Choose the incorrect statement
- An SN1 reaction proceeds with inversion of configuration
- An SN2 reaction proceeds with stereochemical inversion
- An SN2 reaction follows second order kinetics
- The reaction of tert-butyl bromide with OH– follows first order kinetics
- Which of the statements is not true for the isomeric compounds ethylene chloride and ethylidene chloride?
- Both react with aqueous KOH to give the same product
- Both react with alcoholic KOH to give the same product
- They are derivatives of ethene
- They respond to Beilstein’s test
- The reagent for the following conversion is
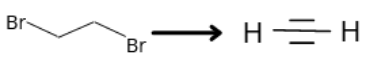

1 . Alcoholic KOH
2. Alcoholic KOH followed by NaNH2
3. Aqueous KOH followed by NaNH2
4. Zn/CH3OH
- Which of the following halogen exhange reaction will occur?
- R-I + NaCl
- R-F + KCl
- R-Cl + NaI
- CH3-F + AgBr
- Which of the following groups are ortho and para directing?
- -CHO
- -NHCOCH3
- -CN
- -SO3H
- The trade name of trochloroethylene is
- Freon
- Westron
- Westrosol
- DDT
- The IUPAC name of the compound shown below is


1 . 2-bromo-6-chlorocyclohex-1-ene
2. 6-bromo-2-chlorocyclohexene
3. 3-bromo-1-chlorocyclohexene
4. 1-bromo-3-chlorocyclohexene
10. The increasing order of hydrolysis of the following compounds is
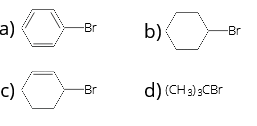
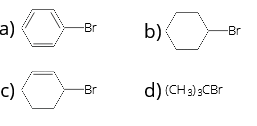
1 . a < d < b < c 2. a < b < c < d
3. a < b < d < c 4. d < c < b < a
Answers (1-10)
1 . (3)
The addition of Cl and I radicals to alkanes in an anti-Markovnikov’s fashion is an endothermic reaction and therefore counterproductive. Without the presence of peroxide, all hydrogen halides will add according to the Markovnikov rule.
2. (4)
Carbylamine reaction takes place
C6H5NH2 + CHCl3 + 3KOH → C6H4NC + 3KCl + 3H2O
As isocyanide is formed, an offensive smell is given out.
3. (1)
SN1 reaction proceeds with racemisation and retention of configuration.
4. (1)
CH2(Cl)-CH2(Cl) + aq KOH → CH2(OH)-CH2(OH)
Ethylene dichloride
CH3-CH(Cl)2 + aq KOH → CH3-CHO
Ethylidene chloride
5. (2)


6. (3)
Bromides and chlorides are insoluble in acetone and hence they can’t form a solution with acetone to react with alkyl halides. Sodium iodide, however is soluble in acetone thus forms a solution with acetone and reacts with alkyl halides, thus undergoing Finkelstein reaction
R-X + NaI → R-I + NaX
7. (4)
-SO3H is an ortho and para directing group.
8. (3)
The trade name of trichloroethylene is westrosol.
9. (3)
3-bromo-1-chlorocyclohexene
10. (2)
a < b < c < d
Click Below To Learn Physics Term 1 Syllabus Chapter-Wise MCQs
Click Below To Learn Chemistry Term-1 Syllabus Chapters MCQs
- Chapter-1: Solid State MCQ
- Chapter-2: Solution MCQ
- Chapter-7: P-Block Element MCQ
- Chapter 10: Haloalkanes and Haloarenes MCQ
- Chapter 11: Alcohols Phenols and Ether MCQ
- Chapter-14: Biomolecule MCQ
Haloalkanes and Haloarenes MCQ (11-20)
- On being warmed with silver powder, chloroform gives
- C6H6
- C2H4
- C2H2
- CH3Cl
- The addition of propene with HOCl proceeds via the additions of
- H+ in the first step
- Cl– in the first step
- OH– in the first step
- Cl+ and OH– in a single step
- Chlorobenzene can be obtained from benzenediazonium chloride by
- Friedel-Crafts reaction
- Sandmeyer reaction
- Wurtz reaction
- Fittig reaction
- Which of the following reactions cannot be used for the preparation of alkyl halides?
- CH3CH2OH + HCl →
- CH3CH2OH + HCl + anhy. ZnCl →
- (CH3)3COH + HCl →
- (CH3)2CHOH + HCl →
- Butyronitrile can be prepared by heating
- Propyl alcohol with KCN
- Butyl alcohol with KCN
- Propyl chloride with KCN
- Butyl chloride with KCN
- For a given alkyl group, the boiling point of alkyl halides follows the order
- RCl > RBr > RI
- RI > RBr > RCl
- RI > RCl > RBr
- RBr > RI > RCl
- Which of the following is the most reactive towards nucleophilic substitution reaction?


1 . (a) 2. (b) 3. (c) 4. (d)
- Addition of HBr gives the same product in the presence or absence of peroxide when alkene is
- 1-butene
- 2-methylpropene
- 1-propene
- 2-butene
- Br has a low reactivity in CH2CHBr because
- The C-Br bond has a partial triple bond character
- Of the +M effect of bromine
- Br is electronegative
- None of the above
- Which of the following is the most suitable for the preparation of n-propylbenzene?
- Friedal-Crafts Alkylation
- Wurtz Reaction
- Wurtz-Fittig Reaction
- Grignard Reaction
Answers (11-20)
11. (3)
2CHCl4 + 6Ag → C2H2 (ethyne) + 6 AgCl
12. (2)
Alkenes undergo electrophilic addition reactions. HOCl on self ionisation produces Cl+ which attacks 1st.
HOCl + HOCl → H2O + OCl– + Cl+
13. (2)
The formation of chlorobenzene by the reaction of benzene diazonium chloride with Cu2Cl2/HCl is known as Sandmeyer’s reaction.
14. (1)
Alkyl halide is prepared by the reaction of an alcohol with HCl in which the -OH group is replaced with Cl.
Primary and secondary alcohols require catalysts such as anhydrous zinc chloride.
Tertiary alcohols do not require such catalysts as they are more reactive.
15. (3)
Butyronitrile can be prepared by heating propyl chloride with KCN.
CH3-CH2-CH2-Cl + KCN → CH3-CH2-CH2-CN
16. (2)
Higher the mass of halogen atoms attached with the hydrocarbon part, higher will be the boiling point. Therefore, as I has the highest mass, then Br and then Cl, the boiling point will decrease as : RI > RBr > RCl
17. (4)
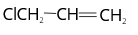
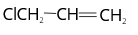
18. (4)
2-butene is a symmetric compound and will produce the same product with HBr with or without peroxide.
19. (2)
Br has a low reactivity in CH2CHBr because of the +M effect of bromine.
20. (3)
The most suitable way for preparing n-propylbenzene is wurtz-fittig reaction.
Haloalkanes and Haloarenes MCQ (21-30)
- What is the hybridisation of carbon that is attached to chlorine atom in n-primary alkyl halide?
- sp3
- sp2
- sp
- sp3d
- The C-X bond is strongest in
- CH3Cl
- CH3Br
- CH3F
- CH3I
- IUPAC name for tertiary butyl iodide is
- 4-iodobutane
- 2-iodobutane
- 1-iodo-3-methylpropane
- 3-iodo-2-methylpropane
- Select the incorrect statement
- 1,4-dibromobutane react with excess of magnesium in ether to generate di-Grignard reagent
- 1,2-dichlorocyclohexane treated with excess of magnesium in ether produces cyclohexane
- Vicinal dihalides undergo dehalogenation to give alkene when heated with Zn dust or Mg
- 1,3-dichloropropane by treatment with Zn dust or Mg forms cyclopropane
- The reaction of benzene with chlorine in the presence of iron gives
- Benzene hexachloride
- Chlorobenzene
- Benzyl chloride
- Benzoyl chloride
- Which is used as an anaesthetic during surgery?
- Chloroquine
- Thyroxine
- Halothane
- Chloramphenicol
- Which among the following halides will acquire colour when exposed to light?
- R-Br and R-Cl
- R-I and R-F
- R-Cl and R-Br
- R-Br and R-I
- Which of the following is used in paint removal?
- CHCl3
- CH2Cl2
- CCl4
- CH3Cl
- Ethyl chloride is converted to butane by
- Kolbe’s synthesis
- Williamson synthesis
- Wurtz reaction
- Gatterman reaction
- Aryl halide is less reactive than alkyl halide towards nucleophilic substitution because
- Of less stable carbonium ion
- Due to large C-Cl bond energy
- Of inductive effect
- Of resonance stabilization and sp3 hybridisation of C attached to halide
Answers (21-30)
21. (1)
n primary alkyl halide is R – CH2 – X
In alkyl halides, halogen is always attached to an sp3 carbon.
22. (3)
The electron pair is pulled towards the halogen in the carbon-halogen bond as the halogen is more electronegative. As fluorine is the most electronegative element, CH3F has the strongest bond and has the shortest bond length.
23. (4)
Tertiary butyl iodide is
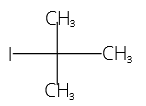

IUPAC Name = 2-iodo-2-methyl propane
24. (3)
Wrong statement – Vicinal dihalides undergo dehalogenation to give alkene when heated with Zn dust or Mg.
25. (2)


26. (3)
Chloroquine – used for malaria
Thyroxine – hormone of thyroid gland
Halothane – anesthetic during surgery
Chloramphenicol – antibiotic for bacterial infections
27. (4)
As we move down the group from fluorine to iodine, molecular size increases.
28. (2)
CHCl3 is used as a solvent and as an anaesthetic.
CCl4 is used as a fire extinguisher.
CH2Cl2 is used as paint removing
CH3Cl is used as a refrigerator
29. (3)
Wurtz reaction :
RX + 2Na + RX → R-R + 2NaX
30. (4)
Aryl halides are less reactive towards nucleophilic substitution reaction as compared to alkyl halides due to resonance stabilisation.
Due to resonance, C-Cl bond acquires partial double bond character and becomes shorter and stronger and can’t be easily replaced by nucleophiles.
Click Below To Learn Chemistry Term-1 Syllabus Chapters MCQs
- Chapter-1: Solid State MCQ
- Chapter-2: Solution MCQ
- Chapter-7: P-Block Element MCQ
- Chapter 10: Haloalkanes and Haloarenes MCQ
- Chapter 11: Alcohols Phenols and Ether MCQ
- Chapter-14: Biomolecule MCQ
Haloalkanes and Haloarenes MCQ (31-40)
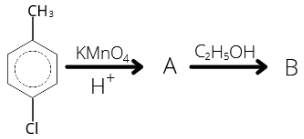
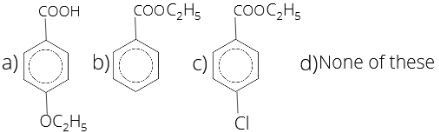
31. Identify B in the following reaction


1 . (a) 2. (b) 3. (c) 4. (d)
- Which metal is used in Wurtz synthesis?
- Barium
- Aluminium
- Sodium
- Iron
- The false statement about alkane is
- This does not perform polymerisation reaction
- This doesn’t gives elimination reaction
- It doesn’t disappear the colour of dilute KMnO4 solution
- It does not decolourize bromine water
- Which of the following is used to decaffeinate coffee?
- Iodoform
- Carbon tetrachloride
- Methylene dichloride
- Chloroform
- Which of the following statements is false about chloroform?
- It is a colourless, sweet-smelling liquid
- It is almost insoluble in water
- It can be used as an inhalational anaesthetic agent
- It is highly inflammable
- Which if the following can not be precipitated with an aqueous solution of AgNO3?
- CHCl3
- KI
- K2SO4
- KCl
- Reaction of chlorobenzene with CH3Cl in presence of AlCl3 gives
- Toluene
- m-chloro toluene
- only o-chloro toluene
- Mixture of ortho and para chlorotoluene
- On nitration, the major product that chlorobenzene gives is
- 1-chloro-4-nitrobenzene
- 1,4-dinitrobenzene
- 1-chloro-3-nitrobenzene
- 2,4,6-trinitrobenzene
- Common name of chloroethane is
- Benzyl chloride
- Vinyl chloride
- Allyl bromide
- None of these
- Which if the following has the highest reactivity?
- CH3Br
- CH3CH2Br
- CH3CH2CH2Br
- CH3CH2CH2CH2Br
Answers (31-40)
31. (3)
CH3-C6H5-Cl + KMnO4 → COOH-C6H5-Cl + C2H5COOH → COOC2H5-C6H5-Cl
32. (3)
Sodium metal is used in the Wurtz reaction.
33. (2)
Alkanes do show elimination reactions.
34. (3)
Methylene dichloride is used to decaffeinate coffee.
35. (4)
Chloroform is not highly inflammable.
36. (1)
In CHCl3, there are no free Cl– ions, hence. It doesn’t form a precipitate with an aqueous solution of AgNO3.
37. (4)
The Cl– group attached is an ortho-para directing group, therefore, it will form ortho and para chloro toluene.
38. (2)
As Cl- is an ortho-para directing group, it will form ortho-para nitrobenzene.


39. (2)
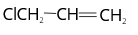
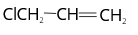
The substituent group attaches to the double bonded or sp2 hybridised carbon. Therefore, it is called vinyl chloride.
40. (1)
Br is an electronegative element. The compound is stable if the carbon chain is of more length and thereby inductive effect increases. CH3Br is less stable and shows higher reactivity because of less inductive effect. Other options have more than one carbon and hence inductive effect increases, stability increases and reactivity decreases.
Haloalkanes and Haloarenes MCQ (31-40)
- Which of the following molecules have the highest dipole moment?
- CH3Cl
- CH2Cl2
- CHCl3
- CCl4
- Which of the following have the highest boiling point?
- 1-chloropentane
- 2-chloropentane
- 3-chloropentane
- All have equal boiling point
- Chlorobenzene and benzene hexachloride are obtained from benzene by the reaction of chlorine, in the presence of
- Direct sunlight and anhydrous AlCl3 respectively
- Sodium hydroxide and sulphuric acid respectively
- Ultraviolet light and anhydrous FeCl3 respectively
- Anhydrous AlCl3 and direct sunlight respectively
- Which of the following can not be prepared by Sandmeyer’s reaction?
- Chlorobenzene
- Bromobenzene
- Iodobenzene
- All of these
- Which of the following reagent is used in the conversion of benzene diazonium chloride to chlorobenzene?
- CuCl2
- Cu2Cl2
- FeCl2
- FeCl3
- The conversion of ethyl bromide to ethyl iodide using sodium iodide and dry acetone, this reaction is known as
- Swarts reaction
- Finkelstein reaction
- Sandmeyer’s reaction
- Stephen reaction
- The vapours of which of the following compound burns with green coloured flame?
- CH3COCH3
- C2H5OH
- CHCl3
- CH3CHO
- At normal temperature iodoform is
- Thick viscous liquid
- Gas
- Volatile liquid
- Solid
- Iodoform on reaction with KOH gives
- CH3CHO
- HCOOK
- CH3COOK
- HCHO
- Triiodomethane has antiseptic property because of
- Liberation of iodoform
- Liberation of free iodide
- Formation of phosgene gas
- None of these
Answers (41-50)
41. (1)
Methane has a zero dipole moment. Replacement of one H- atom by Cl atom increases the dipole moment. The increase in dipole moment is because the bond dipole moment of C-H bond and that of C-Cl bond reinforce each other.
Replacement of another H atom by Cl increases the bond angle due to lone pair-lone pair repulsion between Cl- atoms. This reduces the dipole moment and introduces the third Cl- atom. So when the fourth Cl- atom is introduced, the molecule (CCl4) again becomes symmetrical and the dipole moment reduces to zero. So, CH3Cl has the highest dipole moment.
42. (1)
The more branched a molecule is, the less will be the surface area to interact through van der waal forces and hence, the boiling point will also decrease. Therefore, 1-chloropentane will have the highest boiling point.
43. (4)
Benzene on substitution reaction with chlorine in the presence of anhydrous AlCl3 will form chlorobenzene. Benzene in addition reaction with chlorine in the presence of sunlight will form benzene hexachloride.
44. (3)
Sandmeyer’s reaction is used for the preparation of chlorobenzene and bromobenzene.
45. (2)
Cu2Cl2 is used as a reagent and the reaction is called Sandmeyer’s reaction.
46. (2)
The conversion of ethyl bromide to ethyl iodide using sodium iodide and dry acetone, this reaction is known as Finkelstein reaction.
C2H5-Br + NaI → C2H5I +NaBr
47. (3)
Chloroform burns with green flames gas.
48. (4)
At room temperature, iodoform is a yellow coloured solid.
49. (2)
CHI3 + 4KOH → HCOOK + 3KCl +2H2O
50. (2)
Triiodomethane is known as iodoform. As iodoform comes in contact with the matter of skin it decomposes to give free iodine which acts as an antiseptic. It is used for treating skin infection, cuts, boils etc.
Assertion and Reasoning MCQs
Codes
(a) Both A and R are true and R is the correct explanation of A
(b) Both A and R are true and but R is not a correct explanation of A
(c) A is true but R is false
(d) A is false, but R is true
1. Assertion (A) (CH3)3C-O-CH3 give (CH3)3C-I and CH3OH on treatment with HI.
Reason (R) The reaction occurs by SN1 mechanism.
2. Assertion (A) Hydrogen iodide readily reacts with alkenes to form alkyl halides.
Reason (R) Aqueous hydrohalogen acids are used to prepare alkyl halides from alkenes.
3. Assertion (A) CHCI3 is stored in dark bottles.
Reason (R) CHCl3 is oxidized in dark.
4. Assertion (A) CCI4 is a fire extinguisher.
Reason (R) CCI4 is insoluble in water.
5. Assertion (A) CH2-CH-CH2-X is an example of of a allyl halides.
Reason (R) These are the compounds in which the halogen atom is bonded to an sp² hybridised carbon atom.
6. Assertion (A) Alkylbenzene is not prepared by Friedel-craft alkylation of benzene.
Reason (R) Alkyl halides are less reactive than aryl halides.
7. Assertion (A) Aryl halides cannot be prepared by replacement of of hydroxyl group of phenol by halogen atom.
Reason (R) Phenol react with halogen acids violently.
8. Assertion (A) Exposure of ultraviolet rays to human causes the skin cancer, disorder and disrupt the immune system.
Reason (R) Carbon tetrachloride is released into air it rises to atmosphere and deplete the ozone layer.
9. Assertion (A) The boiling points of alkyl halides decreases in order: RI > RBr > RCI > RF.
Reason (R) The boiling points of alkyl chloride, bromides and iodides are considerably higher than that of the hydrocarbon of comparable molecules mass.
10. Assertion (A) Electron withdrawing groups in aryl halides increase the reactivity towards nucleophilic substitution
Reason (R) 2, 4 Dinitrochlorobenzene is less reactive than chlorobenzene
Assertion and Reasoning MCQ Answers
1. (a)
SN1 reaction occurs in compound that has steric hindrance and (CH3)3-C-OCH3 has very much hindrance to attack by reagents.
2. (c)
Dry gaseous hydrohalogenation acids are better electrophiles. In aqueous solution, H2O acting as nucleophile may produce alcohol.
3. (c)
CHCl3 is stored in dark bottles to avoid reaction in the presence of light. CHCl3 in the presence of light gets oxidised by air.
4. (b)
CCl4 is carbon tetrachloride and is used in fire extinguisher because it is a heavy non-combustible liquid. CCl4 in insoluble in water due to absence of hydrogen atom that can form hydrogen bonding with water.
5. (c)
Allyl halides are the compounds in which the halogen atom is bonded to an sp3 hybridised carbon atom next to carbon-carbon double bond.
6. (c)
Aryl halides are more stable and less reactive due to resonance where the lone pair of electron are in conjugation with a pi bond.
7. (c)
Aryl halides can’t be prepared by replacing hydroxyl group of phenols because the carbon oxygen bond in phenols because has a partial double bond character and is difficult to break being stronger than a single bond.
8. (b)
The ozone layer is depleted as carbon tetrachloride rises into the atmosphere rises into the atmosphere. As the ozone layer depletes, human being are exposed to more UV radiation, which leads to an
9. (b)
The boiling point of the identical hydrocarbon component is determined by the atomic mass of the halogen atom. The boiling point of a halogen atom increases as its mass increases. As a result, the boiling point of halogen atoms lowers as the atomic mass of the halogen atom decreases.
10. (c)
When electron withdrawing groups are present at the ortho/para position, halobenzenes become reactive to nucleuphile substitution reaction. This is evident by the fact that 2,4-dinitrochlorobenzene requires milder hydrolysis condition than chlorobenzene.
Frequently Asked Questions (FAQs)
Q1. Which is more reactive, Haloarenes or Haloalkanes?
Haloarenes are more stable and react less as it is resonance stabilised and has a partial double bond character.
Q2. Why is vinyl chloride is unreactive?
Vinyl chloride (CH2CH2) is stable and unreactive in nucleuphilic suctitution reaction as it is resonance stabilised.
Q3. Why neopentyl bromide undergoes nucleophilic substitution reaction so slowly?
As the bromine is connected to the carbon atom which is surrounded by many alkyl substitutions, it experiences steric hidrance.
Click Below To Learn Chemistry Term-1 Syllabus Chapters MCQs
- Chapter-1: Solid State MCQ
- Chapter-2: Solution MCQ
- Chapter-7: P-Block Element MCQ
- Chapter 10: Haloalkanes and Haloarenes MCQ
- Chapter 11: Alcohols Phenols and Ether MCQ
- Chapter-14: Biomolecule MCQ
Click Below To Learn Physics Term 1 Syllabus Chapter-Wise MCQs
Final Words
From the above article, you have practiced Haloalkanes and Haloarenes MCQ of Class 12 Chemistry Chapter 10. We hope that the above mentioned latest MCQs for Term 1 of Chapter 10 Haloalkanes and Haloarenes will surely help you in your exam.
If you have any doubts or queries regarding the Haloalkanes and Haloarenes Multiple Choice Questions with Answers of CBSE Class 12 Chemistry, feel free to reach us and we will get back to you as early as possible.
Click Below To Learn Physical Education Term-1 Syllabus Chapters MCQs
- Chapter 1: Planning in sports MCQ
- Chapter 2: Sports And Nutrition MCQ
- Chapter 5: Children and Women in Sports MCQ
- Chapter 6: Test and Measurement in Sports MCQ
- Chapter 8: Biomechanics and Sports MCQ