Last updated on July 14th, 2024 at 05:45 pm
Solutions MCQ Chapter 2
Solution is a homogeneous mixture of two or more substances whose compositions can be varied continuously upto the limit of solubility.
Below are some of the very important NCERT Solutions MCQ Class 12 Chemistry Chapter 2 with Answers. These Solutions MCQs have been prepared by expert teachers and subject experts based on the latest syllabus and pattern of CBSE Term 1 examination. We have given these Solutions Class 12 Chemistry MCQs Questions with Answers to help students understand the concept.
MCQ Questions for Class 12 Chemistry are very important for the latest CBSE Term 1 and Term 2 pattern. These MCQs are very important for students who want to score high in CBSE Board, NEET and JEE exam.
We have put together these NCERT MCQ Questions of Solution for Class 12 Chemistry Chapter 2 with Answers for the practice on a regular basis to score high in exams. Refer to these MCQs Questions with Answers here along with a detailed explanation.
Note : – Answers after every 10 questions


Solutions MCQ (1-10)
- Number of components in a binary solution is?
- 2
- 4
- 3
- 1
- Which among the following does not have any unit?
- Molality
- Molarity
- Mole Fraction
- None of the above
- Vapour pressure of dilute aqueous solution of glucose is 730 mm of mercury at 373 K. The mole fraction of solute is?
- 1/10
- 3/76
- 1/76
- 3/28
- With increase in pressure,
- Solubility of solids decreases
- Solubility of solids is not affected
- Solubility of gases increases
- Solubility of gases decreases
- Which of the following substances dissolves in water?
- C6H6
- C4H10
- CH4
- C6H12O6
- Which of the following is a method of expressing the concentration of a solution?
- Osmotic Pressure
- Lowering in freezing point
- Increase in boiling point
- Parts per million
- A 0.0020 molal aqueous solution of an ionic compound CO(NH3)5(NO2)Cl freezes at -0.00732°C. Number of moles of ions which 1 mol of ionic compound produces on being dissolved in water will be (kf = -1.86°C molal-1)
- 1
- 2
- 3
- 4
- Which of the following shows negative deviation from Raoult’s law?
- Water + Nitric Acid
- Benzene + Methanol
- Acetone + Benzene
- Methyl alcohol + Water
- 30 mL of liquid R and 20 mL of liquid S are mixed to form a solution of 50 mL. Then the solution is __________?
- Non-ideal with positive deviation
- Non-ideal with negative deviation
- Ideal Solution (.)
- Cannot be predicted
- PCl5 (g) ⇌ PCl3 (g) + Cl2 (g). In the reaction, at equilibrium condition mole fraction of PCl5 is 0.2 and mole fraction of Cl2 is 0.4. What is the mole fraction of PCl3?
- 0.5
- 0.6
- 0.4
- 0.3
Answers (1-10)
1 . (1)
A binary solution is made by mixing two different species.
2. (3)
Mole fraction is the ratio of the number of moles of a particular component to the total number of moles of the solution. Therefore, it is unitless.
3. (2)
Vapour pressure of water = 760 mm
Vapour pressure of urea = 730 mm
We know,
(P°-P)/P° = Mole fraction = (760-730)/760 = 3/76
4. (3)
According to Henry’s law, solubility of gas is directly proportional to the pressure of the gas present above the solution’s surface.
5. (4)
Glucose (C6H12O6) dissolves in water as both are polar in nature (like dissolves like).
6. (4)
Parts per million is a method of expressing the concentration of a solution
7. (2)
ΔTf = i x kf x m
0.00732 = i x 1.86 x 0.002
i = 1.967 ≅ 2
8. (1)
Water + Nitric Acid shows negative deviation from Raoult’s law
9. (3)
30 mL + 20 mL = 50 mL
Final solution’s volume is also 50 mL.
As the change in volume is zero, therefore it forms an ideal solution.
10. (3)
Mole fraction’s sum is always equal to 1.
Mole fraction of PCl5 + Mole fraction of Cl2 + Mole fraction of PCl3 = 1
0.4 + 0.2 + Mole fraction of PCl3 = 1
Mole fraction of PCl3 = 1 – 0.6 = 0.4
Click Below To Learn Physics Term 1 Syllabus Chapter-Wise MCQs
Click Below To Learn
Chemistry Term-1 Syllabus Chapters MCQs
- Chapter-1: Solid State MCQ
- Chapter-2: Solution MCQ
- Chapter-7: P-Block Element MCQ
- Chapter 10: Haloalkanes and Haloarenes MCQ
- Chapter 11: Alcohols Phenols and Ether MCQ
- Chapter-14: Biomolecule MCQ
Solutions MCQ (11-20)
- In case an electrolyte dissociates in the solution, the van’t hoff factor i is?
- >1
- <1
- 1
- >1 or <1
- Dissolving 120 g of urea (molecular weight 60 g mol-1) in 1000 g of water gave a solution of density 1.15 g mL-1. The molarity of the solution is
- 1.78 M
- 2.00 M
- 2.05 M
- 2.22 M
- Which has the highest freezing point at 1 atm?
- 0.1 molal NaCl solution
- 0.1 molal BaCl2 solution
- 0.1 molal sugar solution
- 0.1 molal FeCl3 solution
- Which of the following conditions is not satisfied by an ideal solution?
- ΔHmix = 0
- ΔSmix = 0
- ΔVmix = 0
- Raoult’s law is obeyed
- A substance trimerizes when dissolved in a solvent. What is the Van’t Hoff factor (assume 100% association)?
- 1
- 2
- 3
- 1/3
- An azeotrope solution of two liquids has boiling point lower than that of either of them if it
- Shows a negative deviation from Raoult’s law
- Shows a positive deviation from Raoutl’s law
- Shows no deviation from Raoult’s law
- Is saturated
- Molarity of 900 g of water is?
- 50 M
- 55.5 M
- 5 M
- 5.55 M
- Increase in the temperature of an aqueous solution will cause
- Decrease in molality
- Decrease in molarity
- Decrease in mole fraction
- Decrease in %w/w
- Water boils at 96°C at a certain place. What is the amount of NaCl to be added to 1 L of water so that it boils at 100°C (Kb for water is 0.52°C molal-1
- 450 g
- 125 g
- 225 g
- 250 g
- An aqueous solution of methanol in water has vapour pressure
- Equal to that of water
- Equal to that of methanol
- More than that of water
- Less than that of water
Answers (11-20)
11. (1)


After dissociation, the number of molecules increases. Therefore i > 1
12. (3)
Total mass of the solution = Mass of urea given + Mass of water given = 1000 + 120 = 1120 g
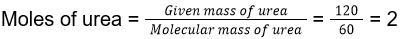
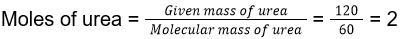




13. (3)
As lowering of freezing point is a colligative property, the increase in number of particles will further lower the freezing point.
NaCl, BaCl2 and FeCl3 will dissociate into 2, 3 and 4 particles respectively (As they are strong electrolytes and have 100% dissociation). Glucose will not dissociate into anything.
Therefore, as the number of dissociated particles is the lowest in the glucose solution, it will have the highest freezing point and the FeCl3 solution will have the lowest freezing point.
14. (2)
An ideal solution obeys Raoult’s law and has ΔHmix = 0 and ΔVmix = 0.
15. (4)
Van’t Hoff factor (i) = 1 – a + (a/n)
As there is 100% dissociation, a = 1
n = 3, i = 1/3
16. (2)
In case of positive deviation from Raoult’s law, the observed vapour pressure is higher than the ideal vapour pressure and boiling point of azeotrope becomes lower than either of pure liquid.
17. (2)
Volume of 900 g of water = 900 mL
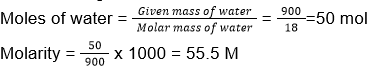
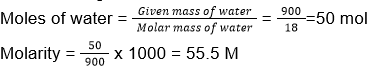
18. (2)
Increase in temperature increases the volume. As molarity is inversely proportional to the volume, so there will be a decrease in the molarity.
19. (3)
ΔTb = i x kb x m
ΔTb = 100 – 96 = 4
NaCl has i = 2
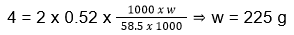
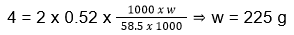
20. (3)
The vapour pressure of aq. methanol solution is more than H2O and less than methanol. This is due to the solute-solvent interaction more due to the formation of H-bonds than methanol but less in water.
Solutions MCQ (21-30)
- If an aqueous solution of glucose is allowed to freeze, then crystals of which will separate out first?
- Water
- Glucose
- Both 1 and 2
- None of these
- Henry’s law constant for the solubility of N2 gas in water at 298 K is 1.0 x 105 atm. The mole fraction of N2 in air is 0.8. The number of moles of N2 from air dissolved in 10 mol of water at 298 K and 5 atm is
- 4.0 x 10-4
- 4.0 x 10-5
- 5.0 x 10-4
- 4.0 x 10-6
- At 80°C, the vapour pressure of pure liquid A is 520 mm Hg and that of pure liquid B is 1000 mm Hg. If a mixture of solutions A and B boils at 80°C and 1 atm pressure, the amount of A in the mixture is (1 atm = 760 mm Hg)
- 52 mol%
- 34 mol%
- 48 mol%
- 50 mol%
- A solution showing a large positive deviation from ideal behavior has
- Higher boiling point that both the components
- ΔHmix is positive
- ΔHmix is negative
- None of the above
- Which of the following colligative properties can help to determine the molar mass of a protein with the greatest precision?
- Osmotic pressure
- Depression in freezing point
- Elevation in boiling point
- Relative lowering of vapour pressure
- At a temperature, total vapour pressure in Torr of a mixture of volatile components A and B is given by p = 120 – 75 xB hence vapour pressure of pure A and B, respectively (in Torr) are
- 120 and 75
- 120 and 195
- 75 and 45
- 120 and 45
- The osmotic pressure of a decinormal solution of BaCl2 at 27°C showing 80% degree of ionisation is
- 3.20 atm
- 4.20 atm
- 5.20 atm
- 6.40 atm
- Which of the following statements is incorrect?
- Gibbs energy change of dissolution of a gas may be +ve or -ve
- Mole fraction of gas in the solution is directly proportional to the partial pressure of the gas above the solution
- Volume of gas dissolved measured at the pressure used is independent of the pressure of gas
- Solubility of gas is always an exothermic process
- The values of observed and calculated molecular masses of calcium nitrate are , respectively, 65.6 g mol-1 and 164 g mol-1. The degree of dissociation of calcium nitrate will be
- 25%
- 50%
- 75%
- 60%
- Equal volumes of ethylene glycol (molar mass = 62 g mol-1) and water (molar mass = 18 g mol-1) are mixed. The depression in freezing point of water is (given kf of water = 1.86 K molal-1 and specific gravity of ethylene glycol is 1.11)
- 0.0033 K
- 0.333 K
- 3.33 K
- 33.3 K
Answers (21-30)
21. (1)
Freezing point is the temperature at which the liquid and the solid form of the substance are in equilibrium and have the same vapour pressure. Due to lower vapour pressure of the solution, the solid form of the solution separates out at a lower temperature. When solid is the solute, it’s the solvent that freezes. Therefore, the water will separate first.
22. (1)
According to Henry’s law,
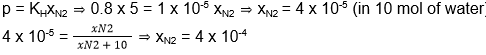
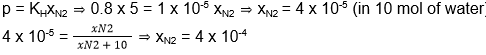
23. (4)
Ptotal = p°AxA + p°BxB⇒ 760 = 520xA + p°B(1-xA) ⇒ xA = 0.5
24. (3)
A solution showing a large positive deviation from ideal behavior has ΔHmix negative.
25. (1)
Osmotic pressure is used for the determination of molar mass of proteins as they are not stable at high temperature and polymers have poor stability.
26. (4)
Ptotal = 120 – 75xB = p°AxA + p°BxB
But, xA = 1 – xB
Ptotal = 120 – 75xB = p°A(1-xB) + p°BxB = p°A – (p°A – p°B)xB
p°A = 120, p°B = 120 – 45 = 75
27. (4)
i = 1 + ɑ(n-1)
ɑ = 0.8, n=3, i=2.6
Decinormal solution ⇒ C = 0.1M
π = i x C x R x T
π = 2.6 x 0.1 x 0.082 x 300 = 6.4 atm
28. (1)
Dissolution of a gas is an exothermic process. Gibbs free energy of an exothermic process is always negative.
29. (3)
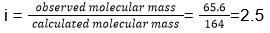
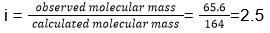


30. (4)
Mass = Density x Volume
Mass(ethylene glycol) = 1.11 x V
Mass(water) = 1.00 x V


ΔTf = i x kf x m


Click Below To Learn
Chemistry Term-1 Syllabus Chapters MCQs and Assertion/Reasoning
- Chapter-1: Solid State MCQ
- Chapter-2: Solution MCQ
- Chapter-7: P-Block Element MCQ
- Chapter 10: Haloalkanes and Haloarenes MCQ
- Chapter 11: Alcohols Phenols and Ether MCQ
- Chapter-14: Biomolecule MCQ
Solutions MCQ (31-40)
- Vapour pressure of a dilute aqueous solution of glucose is 750 mm Hg at 373 K. The mole fraction of the solute is
- 1/10
- 1/(7.6)
- 1/35
- 1/76
- Solutions which distill without change in composition liquid and vapour phase are called
- Amorphous
- Azeotropic mixture
- Supersaturated
- Ideal
- Depression in freezing point of 0.01 molal aqueous acetic acid acid solution is found to be 0.02046°C. One molal urea solution freezes at -1.86°C. Assuming molarity equal to molality, pH of the acidic solution is
- 2
- 3
- 3.2
- 4.2
- A 5% solution of cane sugar (molar mass = 342 g mol-1) is isotonic with a 1% solution of a substance X. The molecular weight of X is
- 34.2
- 171.2
- 68.4
- 136.8
- The molal lowering of vapour pressure for water at 100°C will be
- 13.43 mm Hg
- 1.343 mm Hg
- 23.43 mm Hg
- 234.3 mm Hg
- Which will form maximum boiling azeotrope?
- HNO3 + H2O
- C2H5OH + H2O
- C6H6 + C6H5CH3
- None of the above
- Which of the following statements is not correct for an ideal binary liquid solution AB (XA = mole fraction of A in vapour phase; XB = mole fraction of B in vapour phase)
- A plot between ptotal and XB is linear
- A plot between ptotal and XA is linear
- A plot between 1/ptotal and XA is linear
- A plot between ptotal and 1/XA is linear
- If two substances A and B have p°A : p°B = 1 : 2 and have mole fraction in solution 1 : 2 and have mole fraction of A in vapours is
- 0.33
- 0.25
- 0.52
- 0.20
- Van’t Hoff factors x, y and z for association, dissociation and no change of solute in the solution, respectively, are in the order
- x < y < z
- x > z > y
- x < z < y
- x > y > z
- There is 100 mL of 0.1 M KCl solution. To make it 0.2 M, we have to
- Evaporate 50 mL of the solution
- Add 0.01 mol of KCl
- Use both 1 and 2
- Use neither 1 nor 2
Answers (31-40)
31. (4)
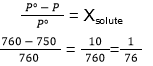
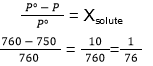
32. (2)
Solutions which distil without change in composition of liquid and vapour phase are called azeotropic mixtures.
33. (2)
ΔTf = i x kf x m
Molarity = molality
Kf = 1.86
0.0204 = 1.86 x i x 0.01
i = 1.1
CH3COOH → CH3COO- + H+
C(1-ɑ) Cɑ Cɑ
i = 1 +ɑ
ɑ = 0.1
[H+] = Cɑ = 0.01 x 0.1 = 0.001
pH = -log[H+] = 3
34. (3)
5% solution means 5g of solute is present in 100g of water
Density of water is 1g/cm3 so 100 g of water is equal to 100mL of water
Mass of solute = 5g
Volume of solution = 100 mL
Molar mass of solute = 342g
For substance X, molar mass = M
Given mass of solute
As mentioned in the question, solution of X and solution of cane sugar are isotonic with each other, i.e,


35. (1)
Lowering of vapour pressure = XA
1 molal aqueous solution means 1 mole of solute in 1000 mL of water
Density of water = 1g/mL
1 mL of water = 1 g
1000 mL of water = 1000 g
No. of moles of water = 55.56XA = nA/(nA + nB) = 0.01768 atm or 13.43 mm Hg
36. (1)
HNO3 + H2O is a maximum boiling azeotrope and shows negative deviation from Roult’s law.
37. (c)
Plot between 1/ptotal and XA is linear is a wrong statement
38. (4)
p°A/p°B = 1/2
p°A = 1, p°B = 2
nA = 1, nB = 2
PA = xA x p°A = (⅓) x 1 = ⅓
PA = xA x p°A = (⅔) x 2 = 4/3
PA = xA x PT


39. (3)
Van’t Hoff factor is the greatest for dissociation and lowest for association
40. (1)
M1V1 = M2V2
100 x 0.1 = X x 0.2
X = 50 mL
We have to reduce the volume of the solution by 50 mL. Therefore, it can be done by evaporating the solution.
Solutions MCQ (41-50)
- A substance is deliquescent if its vapour pressure
- Is less than that of water vapour in the air
- Is more than that of water vapour in the air
- Is equal to that of water vapour in the air
- Is equal to atmospheric pressure
- A pressure cooker reduces cooking time because
- The heat is more evenly distributed
- The higher pressure tenderizes the food
- The boiling point of water inside the cooker is elevated
- The boiling point of water inside the cooker is depressed
- The mass of glucose that should be dissolved in 50g of water in order to produce the same lowering of vapour pressure as produced by dissolving 1 g of urea in the same quantity of water is
- 1 g
- 3 g
- 6 g
- 8 g
- Molarity of liquid HCl, if density of the solution is 1.17 g cm-3, is
- 36.5
- 18.25
- 42.10
- 32.05
- Van’t Hoff factor for 0.1 M ideal solution is
- 1
- 0.1
- -0.01
- None of the above
- Which of the following remains independent of temperature?
- Molality
- Molarity
- Formality
- Normality
- The depression in freezing point for 1 M urea, 1 M glucose and 1 M NaCl are in the ratio
- 1 : 2 : 3
- 3 : 2 : 2
- 1 : 1 : 2
- None of the above
- A liquid is in equilibrium with its vapour at its boiling point. On an average, the molecules in the two phases have equal
- Intermolecular force
- Potential energy
- Total energy
- Kinetic energy
- When mercuric iodide is added to the aqueous solution of potassium iodide, the
- Freezing point rises
- Freezing point decreases
- Freezing point remains the same
- Boiling point doesn’t change
- Which of the following are homogeneous in nature? a) Ice b) Wood c) Soil d) Air
- a and b
- b and d
- a and d
- c and d
Answers (41-50)
41. (1)
A process by which a substance absorbs moisture from the atmosphere till it gets dissolved in the absorbed water and forms a solution is called deliquescence. It occurs only when vapour pressure is less than that of water vapour in the air.
42. (3)
The boiling point of water inside the cooker gets elevated due to which the cooking time reduces.
43. (3)
(Δp)glucose = (Δp)urea
(xB)glucose = (xB)urea
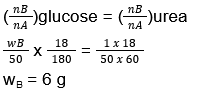
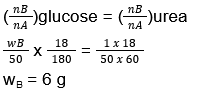
44. (4)
Density = 1.17 g cm-3 = 1170 g/L
Molar mass of HCl = 36.5


45. (1)
Non-electrolytes form ideal solution having van’t hoff factor equal to 1
46. (1)
Molality depends inversely on mass which remains unchanged with the change in temperature.
47. (3)
iurea = 1
iglucose = 1
iNaCl = 2
Ratio = 1 : 1 : 2
48. (4)
As temperature will be the same at the boiling points, kinetic energies will be the same as it is directly proportional to the temperature
49. (1)
As HgI reacts with KI to form K2[HgI4], the number of ions decreases which decreases the van’t hoff factor. This also results in the decrease of freezing point as it is directly proportional to the van’t hoff factor. Thus, the freezing point is raised.
50. (3)
Air and ice are homogeneous in nature as they have the same amount of their components throughout.
Assertion And Reasoning MCQ
Codes
(a) Both A and R are true and R is the correct explanation of A
(b) Both A and R are true and but R is not a correct explanation of A
(c) A is true but R is false
(d) A is false, but R is true
1. Assertion (A) The concentration of pollutants in water or atmosphere is often expressed in terms of PPM.
Reason (R) Concentration in parts per million can be expressed as mass to mass volume to volume and mass to volume.
2. Assertion (A) 0.1 M solution of KCl has greater osmotic pressure than 0.1 M solution of glucose at same temperature.
Reason (A) In solution KCl dissociates to produce more number of particles.
3. Assertion (A) When a solution is separated from the pure solvent by a semipermeable membrane, the solvent molecules pass through the it from pure solvent side to the solution side.
Reason (R) Diffusion of solvent occurs from a region of high concentration solution to a region of low concentration solution.
4. Assertion (A) In solution, amalgam of Mercury with sodium is an example of solid solutions.
Reason (A) Mercury is solvent and sodium is solute in the solution.
5. Assertion (A) Molarity of a solution in liquid state changes with temperature.
Reason (R) The volume of a solution changes with changes in temperature.
6. Assertion (A) Pressure have any effect on solubility of solids in liquid.
Reason (A) Solids and liquids are not incompressible.
7. Assertion (A) Elevation in boiling point is a colligative property.
Reason (R) Elevation in boiling point is directly proportional to molarity.
8. Assertion (A) Azeotropic mixtures are not formed only by non-ideal solutions and they may have boiling points either greater than both the components or less than both the components.
Reason (R) The composition of the vapour phase is not same as that of liquid phase of azeotropic mixture.
9. Assertion (A) At equilibrium, vapor phase will not be always reach in component which is more volatile.
Reason (R) The composition of vapour phase in equilibrium with the solution is not determined by the partial pressure of the components.
10. Assertion (A) An ideal solution obeys Henry’s law.
Reason (R) In an ideal solution, solute-solute as well as solvents-solvent interaction are similar to solute-solvent interaction.
Assertion And Reasoning MCQ Answers
1 . (d)
When a solute is present in trace quantities, it is convenient to express concentration in PPM.
2. (a)
KCl can dissociate in water but glucose does not. Due to dissociation of ions, solution exhibit higher colligative property.
3. (c)
With the semipermeable membrane in place, and if one compartment contains the pure solvent, this can never happen; no matter how much liquid flows through the membrane, the solvent in the right side will always be more concentrated that that in the left side.
4. (c)
Amalgam of mercury with sodium is an example of liquid in solid type solid solution. Here, mercury (liquid metal) acts as solute and sodium as solvent.
5. (a)
Molarity is the number of moles of solute dissolved per litre of solution. Molarity changes as temperature changes as the volume of the solution changes.
6. (a)
Liquid and solids exhibit practically no change of solubility with changes in pressure. Gases as might be expected , increase in solubility with an increase in pressure.
7. (c)
Elevation in boiling point is directly proportional to molality.
8. (b)
Non-ideal solutions with positive deviation, boils at a lower temperature than the components, while those with negative deviation, boil at a higher temperature.
9. (d)
At equilibrium, the vapor phase will always be rich in volatile components. The higher liquids vapor pressure is at a given temperature the higher its volatility and the lower the liquid’s typical boiling point. The partial pressure of components determines the composition of the vapor phase in equilibrium with the solution.
10. (d)
An ideal solution obeys Raoult’s law.
Case Study Based MCQs
1. Read the passage given below and answer the following questions
The vapour pressure of a solvent decreases when a non volatile component is dissolved in the liquid phase. The depression of the vapour pressure of the solution (at constant temperature) results in rise of the the boiling point of the solution (at constant pressure).
The elevation in boiling point being a colligative property, depends on the concentration of the dissolved particles and on the nature of solvent. In dilute solution it is observed to be relatively independent of the nature of the solute
Codes
(a) Both A and R are true and R is the correct explanation of A
(b) Both A and R are true and but R is not a correct explanation of A
(c) A is true but R is false
(d) A is false, but R is true
i) Assertion (A) At boiling point, vapour pressure of a liquid is equal to the atmospheric pressure
Reason (R) Vapour pressure of a liquid decreases with a liquid
ii) Assertion (A) Blood cells collapse when suspended in saline water, having more concentration compared to fluid inside the blood cell
Reason (R) Solvent molecules always flow from higher concentration to lower concentration
iii) Assertion (A) When NaCl is added to water a depression in freezing point is observed
Reason (R) The lowering of the vapor pressure of a solution causes depression in the freezing point compared to the pure solvent
iv) Assertion (A) Colligative property is used to determine the molecular mass of particles
Reason (R) Colligative properties depend upon number of solute particles in solution irrespective of the nature
v) Assertion (A) Colloidal solution show colligative properties
Reason (R) Colloidal particles are large in size
2. Read the passage given below and answer the following question
At 298K, the vapour pressure of pure benzene. C6H6 is 0.256 bar and the vapour pressure of pure toluene C6H5CH3 is 0.095 bar. Two mixtures are prepared as follows:
(I) 7.8g of C6H6 + 9.2g of toluene
(II) 3.9g of C6H6 + 13.8g of toluene
The following questions are multiple choice questions. Choose the most appropriate answer
i) The total vapour pressure (bar) of solution 1 is
- 0.128
- 0.174
- 0.198
- 0.258
ii) Which of the given solutions have higher vapour pressure
- I
- II
- Both have equal vapour pressure
- Cannot be predicted
iii) Mole fraction of benzene is vapour phase in solution I is
- 0.128
- 0.174
- 0.734
- 0.266
iv) Which of the following statement is/are correct
(a) Mole fraction of toluene in vapour phase is more in solution (I)
(b) More fraction of toluene in vapour phase is less than in solution (I)
(c) More fraction of benzene in vapour phase is less in solution (I)
- Only b
- Only a
- a and c
- b and c
v) Solution (I) is an example of a/an
- Ideal solution
- Non ideal solution with positive deviation
- Non ideal solution with negative deviation
- Can’t be predicted
Case Study Based MCQ Answers
- (i) b (ii) c (iii) a (iv) b (v) (b)
- (i) 2 (ii) 1 (iii) 3 (iv) 1 (v) 1
Click Below To Learn Physics Term 1 Syllabus Chapter-Wise MCQs
Click Below To Learn
Chemistry Term-1 Syllabus Chapters MCQs
- Chapter-1: Solid State MCQ
- Chapter-2: Solution MCQ
- Chapter-7: P-Block Element MCQ
- Chapter 10: Haloalkanes and Haloarenes MCQ
- Chapter 11: Alcohols Phenols and Ether MCQ
- Chapter-14: Biomolecule MCQ
Frequently Asked Questions FAQs
Q1. What are the two limitations of Henry’s law?
1. The pressure should be low and the temperature should be high, i.e, the gas should behave like an ideal gas.
2. The gas should not undergo compound formation with the solvent or associate or dissociate in the solvent.
Q2. What is an antifreeze?
An antifreeze is a substance which is added to lower the freezing point of a water based liquid and increase its boiling point. Ethylene glycol is used as an antifreeze.
Q3. Which is better, Molarity or Molality?
Molality is preferred over molarity because molality doesn’t changes with change in temperature as it is inversely proportional to the mass of solvent which doesn’t changes with temperature. Molarity, on the other hand changes with temperature as volume changes with the temperature.
Final Words
From the above article, you have practiced Solutions MCQ of Class 12 Chemistry Chapter 2. We hope that the above mentioned latest MCQs for Term 1 of Chapter 2 Solutions will surely help you in your exam.
If you have any doubts or queries regarding the Solutions Multiple Choice Questions with Answers of CBSE Class 12 Chemistry, feel free to reach us and we will get back to you as early as possible.
Click Below To Learn Physical Education Term-1 Syllabus MCQ
- Chapter 1: Planning in sports MCQ
- Chapter 2: Sports And Nutrition MCQ
- Chapter 5: Children and Women in Sports MCQ
- Chapter 6: Test and Measurement in Sports MCQ
- Chapter 8: Biomechanics and Sports MCQ