Last updated on July 14th, 2024 at 05:36 pm
Metal and Non-metals MCQ
Below are some of the very important NCERT MCQ Questions of Metals and non-metals Class 10 Science Chapter 3 with Answers. These Metals and non-metals MCQ have been prepared by expert teachers and subject experts based on the latest syllabus and pattern of term 1 and term 2. We have given these Metals and non-metals MCQ Class 10 Science Questions with Answers to help students understand the concept.
MCQ Questions for Class 10 Science chapter 3 are very important for the latest CBSE term 1 and term 2 pattern.These MCQs are very important for students who want to score high in CBSE Board.
We have put together these NCERT MCQ Questions of Metals and non-metals MCQ for Class 10 Science Chapter 3 with Answers for the practice on a regular basis to score high in exams. Refer to these MCQs Questions with Answers here along with a detailed explanation.


Metals and Non-metals MCQ 1-25
1. The ability of metals to be drawn into thin wire is known as
(a) Ductility
(b) Malleability
(c) Sonorously
(d) Conductivity
2. The compound obtained on reaction of iron with steam is/are
(a) Fe2O3 (b) Fe3O4
(c) FeO (d) Fe2O3 and Fe3O4
3. Which one of the following properties is not generally exhibited by ionic compounds?
(a) Solubility in water
(b) Electrical conductivity in solid state
(c) High melting and boiling points
(d) Electrical conductivity in molten state
4. Which of the following properties of aluminium are responsible for the same?
(i) Good thermal conductivity
(ii) Good electrical conductivity
(iii) Ductility
(iv) High melting point
(a) (i) and (ii) (b) (i) and (iii)
(c) (ii) and (iii) (d) (i) and (iv)
5. The elements or compounds which occur naturally in the earth crust are known as
(a) Ores
(b) Minerals
(c) Gangue
(d) None of them
6. Silver articles become black on prolonged exposure to air. This is due to the formation of
(a) Ag3N (b) Ag2O
(c) Ag2S (d) Ag2S and Ag3N
7. Which of the following oxide(s) of iron would be obtained on prolonged reaction of iron with steam?
(a) FeO (b) Fe2O3
(c) Fe3O4 (d) Fe2O3 and Fe3O4
8. Which one of the following metals is found in liquid state at room temperature
(a) Na
(b) Fe
(c) Cr
(d) Hg
Click Below For All Class 10 Subjects Sample Papers 2024
9. Predict the metal when 2 mL each of concentrated HCI, HNO3 and a mixture of concentrated HCI and concentrated HNO3 in the ratio of 3:1 were taken in test tubes labelled as A, B and C. A small piece of metal was put in each test tube. No change occurred in test tubes A and B but the metal got dissolved in test tube C respectively.
(a) AI
(b) Au
(c) Cu
(d) Pt
10. An alloy is
(a) an element
(b) a compound
(c) a homogeneous mixture
(d) a heterogeneous mixture
11. This element is very reactive to air and cannot be kept open in air. It reacts vigorously with water. Identify the element from the following
(a) Mg
(b) Na
(c) P
(d) Ca
12. Reaction between X and Y, forms compound Z. X loses electrons and Y gains electrons. Which of the following properties is not shown by Z?
(a) Has high melting point
(b) Has low melting point
(c) Conducts electricity in molten state
(d) Occurs as solid
13. Predict the nature of the element if the electronic configurations of three elements X, Y and Z are X – 2, 8, Y – 2, 8, 7 and Z – 2, 8, 2 respectively?
(a) X is a metal.
(b) Y is a metal.
(c) Z is non-metal.
(d) Y is a non-metal and Z is a metal.
14. What happens when calcium is treated with water?
(i) It does not react with water
(ii) It reacts violently with water
(iii) It reacts less violently with water
(iv) Bubbles of hydrogen gas formed stick to the surface of calcium
(a) (i) and (iv) (b) (ii) and (iii)
(c) (i) and (ii) (d) (iii) and (iv)
15. Which insulating material is used for coating the electrical wires?
(a) Lead (b) Graphite
(c) PVC (d) All can be used
- Unit- I: India And Contemporary World
- Chapter 1: The Rise of Nationalism in Europe
- Unit-II: Contemporary India-II
- Chapter 1: Resources and Development
- Chapter 3: Water Resources
- Chapter 4: Agriculture
- Unit III: Democratic Politics-II
- Chapter 1: Power Sharing
- Chapter 2: Federalism
- Unit IV: Economic
- Chapter 1: Development
- Chapter 2: Sectors of Indian Economy
16. Which of the following properties are not shown in metals?
(a) Electric conductivity
(b) Sonorous in nature
(c) Dullness
(d) Ductility
17. Aluminium foil is used for wrapping food because
(a) it is ductile
(b) it is malleable
(c) it is a good conductor of heat
(d) it is sonorous
18. Of these, the the most ductile metal is
(a) AI (b) Au
(c) Cu (d) Ag
19. Oxides of non-metals are
(a) Acidic
(b) Basic
(c) Neutral
(d) None of these
20. Metal oxides are
(a) Acidic
(b) Basic
(c) Neutral
(d) None of these
21. Food cans are coated with tin and not with zinc because
(a) Zinc is costlier than tin
(b) Zinc has a higher melting point than tin
(c) Zinc is more reactive than tin
(d) Zinc is Iess reactive than tin
22. Which one of the following four metals would be displaced from the solution of its salts by other three metals?
(a) Mg (b) Ag
(c) Zn (d) Cu
23. Mrignayani was doing the experiment of comparing the reactivity of metals in the laboratory. She was given aluminium metal and was told to check reactivity by using four solutions as shown below. She would observe that reaction takes place in
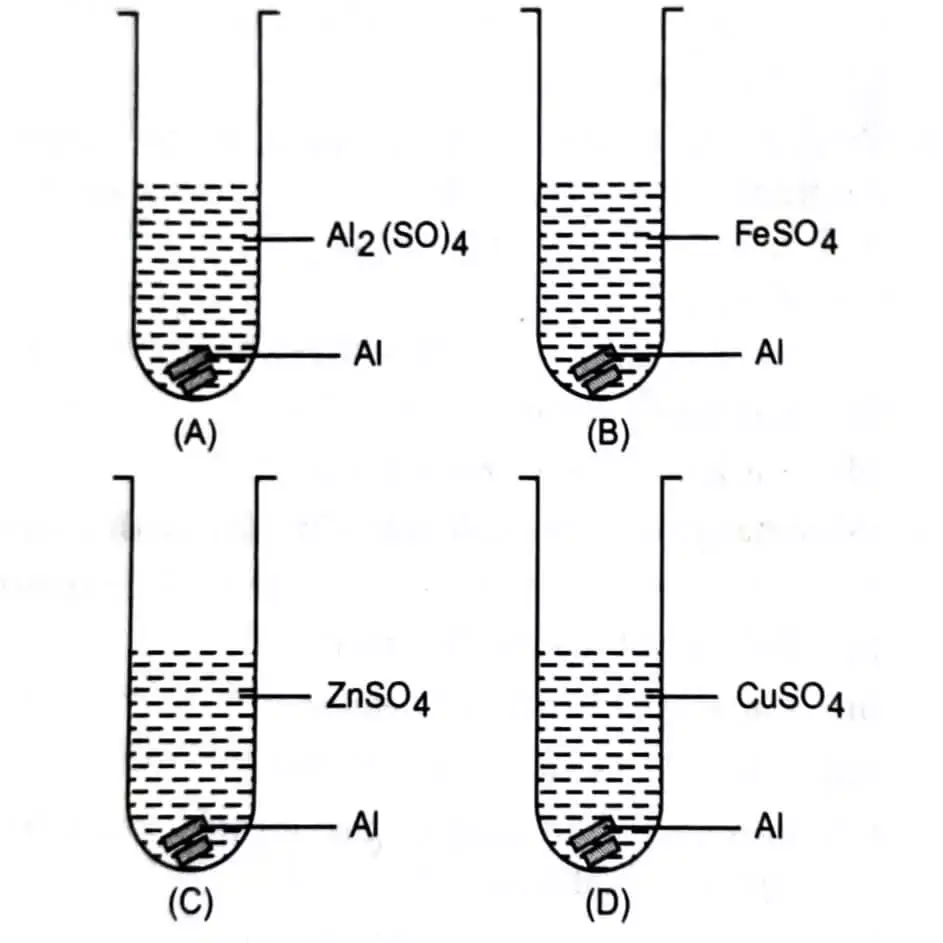

(a) A and B (b) B, C and D
(c) A, C and D (d) C and D
24. Which of the following alloys contain non-metal as one of their constituents?
(a) Brass
(b) Bronze
(c) Steel
(d) Amalgam
25. Which metal is displaced when zinc metal is put in the solution of copper sulphate?
(a) Zinc (b) Copper
(c) Sulphate (d) All of these
MCQ Anwers
1. (a)
Ductility is the property which allows the metals to be drawn into thin wires.
2. (b)
Iron does not reacts with the cold and hot water, but it reacts with steam to form metal oxide and hydrogen.
3 Fe + 4H2O → Fe3O4 + 4H2
3. (b)
Ionic compounds can conduct electricity in aqueous solution or molten state. In solid state, they are non-conductors of electricity because of the absence of free ions.
4. (d)
Good thermal conductivity and high melting point of aluminum are the properties that make it suitable for making cooking utensils. Minerals.
5. (b)
The elements or compounds which occur naturally in the earth crust are known as minerals.
The mineral from which an element can be extracted easily and profitably is called an ore.
The impurities or unwanted substances present in ore such as sand, rock etc is known as gangue.
6. (c)
On prolonged exposure to air silver metal reacts with the hydrogen sulphide gas and forms a coating of silver sulphide (AgS) due to which silver metal loses its shine, turns black and get tarnished.
7. (c)
On prolonged heating with steam iron produces a mixed oxide of iron Fe3O4 with the evolution of hydrogen gas
3Fe + 4H2O → Fe3O4 + 4H2
8. (d)
Except mercury, all the metals are solid at room temperature, Hence, mercury is the only metal which exist in liquid state at room temperature.
9. (b)
Aqua regia which is A mixture of three parts of concentrated hydrochloric acid and one part of concentrated nitric acid (Aqua regia – 3:1 ratio) is given in the test tubes A, B and C to test the metals.
Out of the given options Al, Au, Cu, Pt; gold is a noble metal that cannot dissolve in dilute acids and dissolves only in aqua regia. Hence, the metal that got dissolved in test tube C is gold (Au)
Click Below For All Class 10 Subjects Sample Papers 2024
10. (c)
Suspensions and colloids are heterogeneous mixture whereas an alloy is a homogeneous mixture of two or more metals.
The various properties of a metal can be improved by mixing it with another metal such as melting point, electrical conductivity, resistance to corrosion and strong metals.
Examples of alloys are stainless steel (homogeneous mixture of Fe, Cr, Ni), brass (homogeneous mixture of Cu and Zn), bronze (homogeneous mixture of Cu and Sn) etc.
11. (b)
Element A is sodium as it is soft and can be cut with a knife. It is kept in kerosene because it reacts vigorously when it comes in contact with water or air.
12. (b)
Reaction between compound X and Y forms compound Z. In the reaction, X loses electron and Y gains electron which means compound Z is a crystalline solid as ionic or electrovalent bond is formed between X and Y.
As compound Z is a crystalline solid, it has a high melting point and it conducts electricity in the molten state. Thus, the option which is incorrect is that it has a low melting point.
13. (d)
Electronic configuration of X is 2, 8 = 10 (it is same as the electronic configuration of Neon). Hence, element X is a noble gas element.
Electronic configuration of Y is 2,8,7 = 17 (It is the same as the electronic configuration of chlorine atoms which belongs to the halogen family and is nonmetal). Hence, element Y is non-metal.
Electronic configuration of Z is 2,8 , 2 = 12 (it is the same as the electronic configuration of magnesium atoms which belongs to alkaline earth metals and is a metal). Hence, element Z is a metal.
14. (d)
Calcium metal reacts less violently with water. It produces a hydroxide known as calcium hydroxide (a cloudy white precipitate) and the bubbles of hydrogen gas produced. The hydrogen gas thus produced stuck to the surface of calcium due to which it floats over the water surface
Ca(s) + 2H2O(l) → Ca(OH)2(aq) + H2
Very less amount of heat is produced due to which hydrogen gas formed does catch fire.
15. (c)
16. (c)
Metals are good conductors of electricity. Metals are sonorous in nature which means on striking them they make a ringing sound. Metals are lustrous which means they have shiny appearance. Metals are ductile which means they can be drawn into long thin wires. Hence, the property not shown in metals are dullness.
17. (b)
Aluminium, being a less reactive metal, does not react with food items and does not alter their taste. Moreover, it is highly malleable and can be beaten into very thin foils which are perfect for food wrapping.
18. (b)
Ductility is the property by which metals can be drawn into long thin wires without breaking. Gold, silver and platinum are often drawn into long stands for use in jewellery. However gold has a significantly greater ductility than platinum and it is considered as the earth’s most ductile metals as one ounce of gold could be drawn to a length of 50 miles.
19. (a)
Non-metals react with oxygen to form non-metallic oxide. Non-metallic oxide such as sulphur dioxide and nitrogen dioxide present in air are responsible for acid rain as they react with the moisture present in air and form acidic compounds.
20. (b)
Oxides of metals are basic in nature because they react with water to form metal hydroxides which are alkaline in nature. These metal hydroxides when dissolved in water release OH- ions in solution which will turn red litmus solution to blue. Therefore, non-metallic oxides are acidic in nature.
21. (c)
Food cans are coated with tin and not with zinc because zinc is above the tin in the reactive series and can react with the food items and alter their taste. Hence, due to these reasons food cans are coated with tin and not with zinc.
22. (b)
Silver (Ag) metal would be displaced from the solution of its salts by other three metals because silver is less reactive than magnesium (Mg), zinc (Zn) and copper (Cu),
23. (b)
In the reactivity series alt,jminium ie above zinc, iron and copper which makes it more reactive than those elements. Mrignayni would observe that out of the four solutions, reaction will take place in solution B, C and D. The reactions are as follows:
In Solution B: Aluminium will displace iron from ferrous sulphate solution to form Aluminium sulphate and iron is precipitated
2FeSO4(aq) + 2Al(s) → Al(SO4)3(aq) + 3Fe(s)
In Solution C: Aluminium will displace zinc from zinc sulphate solution to form aluminium sulphate and zinc is precipitated.
In Solution D: Aluminium will displace copper from copper sulphate solution to form aluminium sulphate and copper is precipitated.
24. (c)
Steel is an alloy which contains iron as a metal and carbon as a non-metal.
25. (b)
When zinc metal is put in the solution of copper sulphate, zinc metal will displace copper from its solution and form zinc sulphate. This is because zinc is above copper in the reactivity series and therefore, zinc is more reactive than copper. Also, the blue colour of the solution becomes colourless when zinc is added.
Click Below For All Class 10 Subjects Sample Papers 2024
Very Short Answer Type Question
1. Name the metal which has a low melting point and can melt with the heat of your palm.
2. Name the metal which reacts with a very dilute HNO3 to evolve hydrogen gas.
3. What happens when ZnCO3 is heated in the absence of air? Give the relevant equation.
4. Name one metal which hag a low melting point.
5. Name the metal which is the poorest conductor heat.
6. Name two metals which form the amphoteric oxides.
7. Why is carbon not used for reducing aluminium fro aluminium oxide.
8. Why should sodium hydroxide not be stored in containers?
9. Why does not occur in native state?
10. Why is tungsten used for filament of electric bulb?
Very Short Answer Type Questions Answer
1. Gallium.
2. Magnesium
3. When ZnCO3 is heated in the absence of air, ZnO and CO2 are formed.
4. Cesium.
5. Lead.
6. Aluminium and Zinc
7. Carbon is not used for reducing aluminium from aluminium oxide because auminium reacts more rapidly with oxygen than carbon.
8. Sodium hydroxide should not be stored in a glass container as it reacts with glass.
9. Aluminium does not occur native state because it is a reactive metal, combines with oxygen and readily forms alumina.
10. Tungsten is used for filament of electric bulbs because it has a high melting point and high resistance.
Assertion and Reasoning MCQs
Code
- If both assertion and reason are true and reason is the correct explanation of assertion.
- If both assertion and reason are true, but reason is not the correct explanation of assertion.
- If the assertion is true, but the reason is false.
- If the assertion is false, but the reason is true.
1. Assertion: Sodium is kept immersed in kerosene oil.
Reason: Sodium is a very reactive metal.
2. Assertion: Platinum, gold and silver are used to make jewellery.
Reason: It is because they are very lustrous.
3. Assertion: Copper is used to make hot water tanks and not steel (an alloy of iron).
Reason: Copper does not react with hot water.
4. Assertion: Alloys are commonly used in electrical heating devices like electric iron and heater.
Reason: Resistivity of an alloy is generally higher than that of its constituent metals but the alloys have low melting points than their constituent metals.
5. Assertion: Aluminium is used to make utensils for cooking.
Reason: Aluminium is a highly reactive metal.
6. Assertion: Tungsten is used for filament of electric bulbs.
Reason: It has a high melting point.
7. Assertion: Ionic compounds generally have high melting points.
Reason: It is because they are ionic in nature.
8. Assertion: From metals school bells are made.
Reason: Metals are sonorous.
9. Assertion: Zinc fails to evolve hydrogen gas on reacting with dilute sulphuric acid.
Reason: It gives the NO gas on reaction with ammonia.
10. Assertion: Reactivity series is an arrangement of elements based on their reactivity.
Reason: Reactivity series is used to separate elements based on their reactivity.
Assertion And Reasoning MCQ Answers
1. (1)
Sodium is a very reactive metal. It reacts so vigorously that it catches fire if kept in the open. Therefore, to prevent accidental fires, it is kept immersed in kerosene oil.
2. (1)
Platinum, gold, and silver are used to make jewellery because they are very lustrous. Also, they are very less reactive and do not corrode easily.
3. (3)
Copper does not react with cold water, hot water or steam. However, iron reacts with steam. If the hot water tanks are made of steel (an alloy of iron), then iron would react vigorously with the steam formed from hot water.
4. (2)
An alloy is a combination of a metal and a nonmetal. So it has partial characteristics of metal and non metal. In an alloy the ions are randomly arranged. It does not have a specific arrangement of ions. Hence the flow of electrons in this is more. Features of alloy lead e to increase in reactivity due to free crystal lattice.
5. (2)
Aluminium is a highly reactive metal but it is resistant to corrosion. The reason for the a is that aluminium reacts with oxygen present in air to form a thin layer of aluminium oxide.
This oxide layer is very stable and prevents further reaction of aluminium with oxygen. It is light in weight and a good conductor of heat.
Therefore, Aluminium used to make utensils for cooking.
6. (1)
Tungsten is used for filament of electric bulbs because it has a high melting point and high resistance.
7. (2)
Ionic compounds are made up of positive and negative ions. There is a strong electrostatic force of attraction between them.
A lot of heat energy is required to break this force of attraction and to melt or boil the ionic compound. As a result, ionic compounds have high melting points.
8. (2)
School bells are made up of metals because metals are sonorous.
9. (2)
It is because dil. HNO3 is an oxidising agent therefore, zinc gives NO and not H2 with dil. HNO3.
10. (1)
Reactivity series is an arrangement of elements based on their reactivity in the increasing or the decreasing order and this series is used to separate elements based on their reactivity.
Click Below For All Class 10 Subjects Sample Papers 2024
Case Study Based MCQs
1. Read the passage carefully and answer any four questions.
The chemical reactivity of an element depends upon the atomic structure and its electronic configuration chemical reactivity is shown by all elements which have less than eight electrons in the outermost shell.
Through chemical reactions, atoms of all elements actually try to achieve a completely filled valence shell, Metals have the tendency to loge one more electrons from their valence shell and achieve the nearest noble configuration. This property the metals is called electronegativity. compounds formed by the transfer of electrons from one element to other are known ionic or electrovalent compound.
(i) Three elements A, B and C have their electronic configuration shown below:
A:2, Y: 2,8,7 Z: 2,8,2
Which of the following is correct regarding these elements?
(a) A is a metal
(b) Y is a metal
(c) Z is a non-metal
(d) Y is a non-metal and Z is a metal
(ii) Element S reacts with element T to form a compound C. During the formation of compound C, atoms of S lose one electron whereas T gains one electron each. Which of the following properties is not shown by compound C?
(a) High melting point
(b) Low melting point
(c) Occurrence as solid
(d) Conduction of electricity in the molten state
(iii) The electronic configuration of sodium ion is
(a) 2,8,8
(b) 2,8,2
(c) 2,6
(d) 2,8
(iv) Which of the following represents an electric positive elements
(a) 2,8,8,1
(b) 2,8,8
(c) 2,8,6
(d) 2,7
(v) Choose the incorrect one:
(a) An ionic bond represents sharing of electrons
(b Metals are electropositive
(c) Non-metals are electronegative
(d) Atoms react in order to complete their octet
2. Read the passage carefully and answer any four questions.
An ionic compound is a chemical compound in which ions of elements are held together by ionic bonds. In this type of bond, two oppositely charged ions are held strongly through electrostatic forces.
Metals have loosely bound electrons in their valence shell whereas non-metals need electrons for octet completion and to attain noble gas configuration. The metal thus completely loses an electron and the non-metal accepts it.
By this transfer of electrons, the atoms remain no longer neutral. Cations and anions are formed respectively, Usually, ionic compounds are solids and found in the form of crystals. They have high melting and boiling points.
(i) Which among the following forms a cation?
(a) Oxygen (b) Neon
(c) Potassium (d) Fluorine
(ii) Ionic compounds are soluble in which of the following?
(a) Petrol
(b) Water
(c) Kerosene
(d) Edible oil
(iii) Consider these statements about ionic compounds:
I. They conduct electricity in solid-state
ll. Conduct electricity in solutions.
Ill. They conducts electricity in the molten state.
Choose the correct option
(a) (I) only (b) (II) only
(c) (III) only (d) (II) and (III) only
(iv) Which of the following can change to an anion?
(a) Xenon (b) Iodine
(c) Calcium (d) Magnesium
(v) Identify the incorrect statement-
(a) Ionic compounds are usually brittle
(b) Sharing of electrons is involved in ionic bonds
(c) Common salt is an ionic compound
(d) Ions are fundamental units of ionic compounds
3. Read the chart carefully and answer any four questions.
Metals exhibit their chemical properties as per their electron releasing tendency of atoms. The greater the tendency, the more is the reactivity. Metals react with oxygen, water, hydrogen, acids, etc. They act as reducing agents because they can lose electrons. Some reactions metals undergo are given in this chart:
- Metal + Oxygen → Metal oxide
- Metal + Water → Metal Hydroxide + Hydrogen
- Metal + Acid(dil.) → Metal Salt + Hydrogen
- Metal A + Salt Solution of Metal B → Salt solution of A + B (Displacement)
(i) Some metals react vigorously with oxygen so for safety they are kept in kerosene to prevent accidental fires. Which metals are these?
(a) Phosphorous, Magnesium
(b) Sodium, Potassium
(c) Tin, lead
(d) Calcium, Thallium
(ii) Which of the following pairs will undergo displacement reaction:
(a) Magnesium Chloride and aluminum metal
(b) Silver nitrate solution and copper metal
(c) Ferrous sulphate solution and silver metal
(d) Sodium chloride solution and copper metal
(iii) Identify four metals P, Q, S, T with the hints given below:
(a) P – forms basic oxides
(b) Q – forms amphoteric oxides
(c) S – oxide dissolves in water to form alkali
(d) T – does not react with water
(iv) The metal which does not react with dilute HCI is
(a) Copper (b) Iron
(c) Zinc (d) Sodium
(v) Food cans are coated with tin and not with zinc because:
(a) Zinc is less reactive than tin
(b) Zinc has a higher melting point than tin
(c) Zinc is more reactive than tin
(d) Zinc is costlier than tin
4. Read the following and answer any four questions.
All metals do not react with oxygen at the same rate. Different metals show different reactivities towards oxygen. Almost all metals combine with oxygen to form metal oxides.
Metal oxides are basic in nature but some metal oxides, such as aluminium oxide, zinc oxide, etc show both acidic as well as basic behaviour.
Most metal oxides are insoluble in water but some like Sodium oxide and potassium oxide dissolve in water to produce alkalis.
(i) Arrange the metals in the correct order of their reactivity:
(a) Mg > Al > Zn > Fe
(b) Al > Mg > Fe > Zn
(c) Mg > Zn > Al > Fe
(d) Al > Fe > Zn > Mg
(ii) Why does the magnesium ribbon need to be cleaned before burning it in air?
(a) To increase its efficiency.
(b) To remove the oxide layer from it
(c) To decrease its efficiency
(d) All of these
(iii) What is the reason for the surface of aluminium turning into dull colour after a few days as shown in the figure?


(a) Due to the formation of a stable aluminium oxide layer.
(b) Due to the reaction with atmospheric dirt particles.
(c) Due to its ductile nature.
(d) None of these.
(v) Although metals form basic oxides, which of the following metals form an amphoteric oxide?
(a) Na
(b) Ca
(c) Al
(d) Cu
(vi) Aluminium oxide reacts in the following manner with bases. The resultant product is:
Al2O3 + 2NaOH → 2AICl3 + 3H2O
(a) 2Na
(b) AlO2+H2O
(c) Al2O3 + H2O
(d) None of these
5. Read the following and answer any four questions.
Metals are lustrous, malleable, ductile and are good conductors of heat and electricity. They are the solids at room temperature, except mercury which is a liquid. They can form positive ions by losing electrons to nonmetals.
Metals combine with oxygen to form basic oxides. Different metals have different reactivities with water and dilute acids. Metals above hydrogen in the Activity series can displace hydrogen from dilute acids.
A more reactive metal displaces a less reactive metal from its salt solution. Metals occur in nature free elements or in the form of their compounds.
The extraction of metals from their ores and then refining them for use is known as metallurgy. The surface of some metals, such as iron, is corroded when they are exposed to moist air for a long period of time.
This phenomenon is known as corrosion. Non-metals have properties opposite to that of metals. They are neither malleable nor ductile. They are bad conductors of heat and electricity, except for graphite, which conducts electricity.
(i) Which of the following pairs will give displacement reactions?
(a) MgCl2 solution and aluminium metal.
(b) NaCl solution and copper metal.
(c) FeSO4 solution and silver metal.
(d) AgNO3 solution and copper metal.
(ii) An element reacts with oxygen to give a compound with a high melting point. This compound is also soluble in water. The element is likely to be:
(a) Calcium (b) Silicon
(c) Iron (d) Carbon
(iii) A student placed a Zn rod in the FeSO4 solution. After 10 hours when rod was taken out and it was observed that:
(a) Zn rod became thinner.
(b) Zn rod became thicker due to Iron deposition.
(c) Zn rod remains as it was.
(d) Zn rod has holes.
(iv) Formula of cinnabar is:
(a) HgS (b) HgS2
(c) HgS4 (d) None of these
6. Read the passage carefully and answer any four questions.
| Metal | CuSO4 | ZnSO4 | FeSO4 | AgNO3 |
| A | No change | No change | No change | A coating on metal |
| B | Brown coating | – | Grey deposit | A coating on metal |
| C | No change | No change | No change | No change |
| D | – | No change | No change | Brown deposit |
| E | Brown deposit | New coating | New coating | New coating |
Sample pieces of five metals A, B, C, D, and E are added to the tabulated soluåons separately. The results observed are shown in the table given below. Based on the observations recorded in the table answer the following questions.
(i) Which is the most reactive metal?
(a) B (b) C (c) D (d) E
(ii) Which is the least reactive metal?
(a) A (b) C (c) E (d) B
(iii) Activity series of elements is:
(a) The arrangement of elements in increasing order of reactivity
(b) The arrangement of elements in decreasing order of reactivity
(c) The arrangement of oxides of elements in increasing order of reactivity
(d) None of the above
(iv) Which of the following metal is least reactive:
(a) Zinc
(b) Copper
(c) Silver
(d) Iron
(v) Decreasing order of reactivity is:
(a) A > B > C > D > E
(b) B > E > C > D > A
(c) E > B > D > A > C
(d) D > C > B > E > A
7. From the given table, answer the following questions:
| S. No. | Metals | Non-metals |
| 1. | Lustrous | Non-lustrous |
| 2. | Hard | Soft |
| 3. | Ductile | Non-ductile |
| 4. | Malleable | Non-malleable |
| 5. | Good conductors | Poor conductor |
(i) Give one example each of metals and non-metals.
(ii) Explain ductility.
(iii) Explain malleability.
(iv) What is meant by luster ?
8. A black metal oxide XO2 is used as a catalyst in the preparation of oxygen gas from potassium chlorate.
The oxide XO2 is also used in ordinary dry cells. The metal oxide XO2 cannot be reduced satisfactorily with carbon to form metal X.
(i) Name the metal X
(ii) Name the metal oxide XO2
(iii) Which reducing agent can be used to reduce XO2 to obtain metal X?
(iv) Name another metal which can also be extracted by the reduction of its oxide with the above reducing agent.
9. Nikita took Zn, Al, Cu, Fe, Mg, Na metals and put each metal in cold water and then hot water. She reacted the metal with steam
(i) Name the metal which reacts With cold water.
(ii) Which of the above metals react with steam?
(iii) Name the metal which reacts with hot water.
(iv) Arrange these metals in order of increasing reactivity.
10. Consider the following table given below and answer the questions with reasons.
| S. No. | Metals | Iron (II) Sulphate | Copper (II) sulphate |
| 1. | I | No reaction | Displacement reaction |
| 2. | II | Displacement reaction | Displacement reaction |
| 3. | III | No reaction | No reaction |
| 4. | IV | No reaction | No reaction |
(i) Which metals are more reactive than iron?
(ii) Which metals are more reactive than copper?
(iii) Which of the following is correct?
(a) Metal IV is least reactive.
(b) Metal I is least reactive.
(c) Metal Il is least reactive.
(d) Metal I is most reactive.
(iv) Which metals are least reactive?
Answers
1.
(i) (d) Y is a non-metal and Z is a metal
(ii) (b) Low melting point
(iii) (d) 2,8
(iv) (a) 2,8,8,1
(v) (a) An ionic bond represents sharing of electrons
2.
(i) (c) Potassium
(ii) (b) Water
(iii) (d) Il and Ill only
(iv) (b) Iodine
(v) (b) Sharing of electrons is involved in ionic bonds
3.
(i) (b) Sodium, Potassium
(ii) (b) Silver nitrate solution and copper metal
(iii) (d) P Cu, Q Zn, S K, T
(iv) (a) Copper
(v) (a) Zinc is more reactive than tin
4.
(i) (c)
(ii) (b) To remove the oxide layer from it
(iii) (a) Due to the formation of a stable aluminium oxide layer.
(iv) (c) Al
(v) (b) 2NaAlO2 + H20
5.
(i) (d) AgNO3 solution and copper metal
(ii) (a) Calcium
(iii) (a) Zn rod becomes thinner.
(iv) (a) Hgs
6.
(i) (d) E
(ii) (b) C
(iii) (b) The arrangement of elements in decreasing order of reactivity
(iv) (c) Silver
(v) (c)
7.
(i) Sodium is metal and chlorine is non-metal.
(ii) Ductility is the property of metals in which they are converted into wires.
Click Below For All Class 10 Subjects Sample Papers 2024
- Unit- I: India And Contemporary World
- Chapter 1: The Rise of Nationalism in Europe
- Unit-II: Contemporary India-II
- Chapter 1: Resources and Development
- Chapter 3: Water Resources
- Chapter 4: Agriculture
- Unit III: Democratic Politics-II
- Chapter 1: Power Sharing
- Chapter 2: Federalism
- Unit IV: Economic
- Chapter 1: Development
- Chapter 2: Sectors of Indian Economy
Final Words
From the above article, you have practiced Metals and non-metals MCQ of class 10 Science chapter 3. We hope that the above-mentioned MCQs for term 1 of chapter 3 Science will surely help you in your exam.
If you have any doubts or queries regarding Metals and non-metals MCQ (Multiple Choice Questions) with Answers, feel free to reach us and we will get back to you as early as possible.
Click Below To Learn Physical Education Class 12 Syllabus Chapters MCQs
- Chapter 1: Planning in sports MCQ
- Chapter 2: Sports And Nutrition MCQ
- Chapter 5: Children and Women in Sports MCQ
- Chapter 6: Test and Measurement in Sports MCQ
- Chapter 8: Biomechanics and Sports MCQ